Chemical Collection (page 9)
Chemical discoveries have shaped our world in countless ways, revolutionizing industries and transforming the way we live
All Professionally Made to Order for Quick Shipping
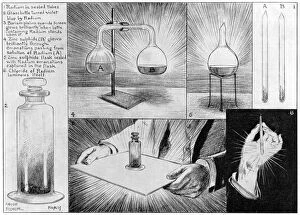
Chemical discoveries have shaped our world in countless ways, revolutionizing industries and transforming the way we live. One of the most significant breakthroughs came in 1869 with Mendeleyev's periodic table, which organized elements based on their properties and paved the way for further scientific advancements. In that same era, another remarkable invention emerged - the Bakelite telephone. This early plastic device marked a turning point in telecommunications technology, showcasing the potential engineering to create innovative materials. But not all chemical reactions bring about positive outcomes. Fire, a powerful force fueled by chemical reactions, can be both destructive and mesmerizing. Its ability to transform matter is awe-inspiring yet serves as a reminder of nature's raw power. Dmitri Mendeleev himself was no stranger to caricatured fame as his contributions to chemistry were widely recognized. His genius lay in organizing elements into groups with similar properties, forever immortalized through humorous depictions of his likeness. Centuries before Mendeleev's time, there was Count of St Germain - a mysterious French alchemist who dabbled in various branches of science including chemistry. Legends surround this enigmatic figure whose pursuit of transmutation captivated many throughout history. The combination of copper and magnesium sulphate (LM) showcases how they can interact to produce stunning visual effects under controlled conditions within laboratories. These experiments provide valuable insights into fundamental principles governing chemical reactions. Advancements continued well into the 20th century when mass spectrometers became indispensable tools for analyzing compounds at an atomic level. Their introduction in 1954 opened new doors for researchers seeking deeper understanding and precise measurements within the realm of chemistry. Chemistry also plays a crucial role beyond laboratory settings; it extends its reach even into medicine. Anesthetics inhibiting ion channels like C015/6718 have revolutionized surgical procedures by providing pain relief during operations while ensuring patient safety remains paramount. Within any laboratory setting, a trusty laboratory clamp is an essential tool.