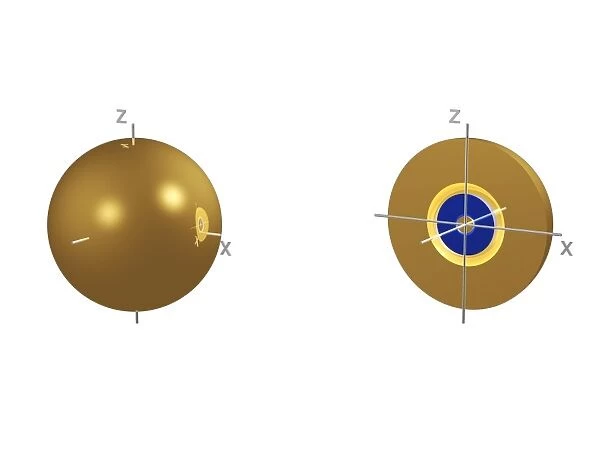
3s electron orbital
![]()

Wall Art and Photo Gifts from Science Photo Library
3s electron orbital
1s electron orbital, computer model. An electron orbital is a region around an atomic nucleus (not seen) in which one or a pair of electrons is most likely to exist. The orbital is seen halved at right to show axes of symmetry. 1s is the lowest energy electron orbital. 1 indicates that it is the lowest energy orbital, ands indicates that it is a single, spherical orbital. The 1s orbital is filled in every element except hydrogen, in which it contains just one electron. This configuration is formally written 1s1. In the next element, helium, the 1s orbital is full, containing two electrons. This is written 1s2
Science Photo Library features Science and Medical images including photos and illustrations
Media ID 6283275
© DR MARK J. WINTER/SCIENCE PHOTO LIBRARY
Atom Atomic Axes Axis Configuration Electron Electron Orbital Mechanical Orbital Orbitals Quantum Mechanics Quantum Physics Shell Shells Spherical Computer Artwork Physical
EDITORS COMMENTS
This print showcases a computer-generated model of the 3s electron orbital, with the additional inclusion of the 1s electron orbital for reference. The image beautifully illustrates an electron orbital as a distinct region surrounding an atomic nucleus, where one or a pair of electrons is most likely to be found. To aid comprehension, the orbital is cleverly halved on the right side to reveal its axes of symmetry. The 1s electron orbital holds significant importance in understanding atomic structure and energy levels. As denoted by its name, it represents the lowest energy level available for electrons within an atom. Its spherical shape signifies that it is a single, symmetrical entity. Interestingly, all elements except hydrogen possess this filled 1s orbital configuration containing just one electron (formally written as 1s1). However, helium breaks this pattern by having two electrons occupying its complete 1s orbital (written as 1s2). This exceptional visual representation combines elements from various scientific disciplines such as chemistry and physics to provide insights into quantum mechanics and atomic behavior. The intricate details captured in this computer artwork shed light on fundamental concepts like shells, orbitals, axes of symmetry while emphasizing their significance in explaining chemical properties and configurations.
MADE IN THE UK
Safe Shipping with 30 Day Money Back Guarantee
FREE PERSONALISATION*
We are proud to offer a range of customisation features including Personalised Captions, Color Filters and Picture Zoom Tools
SECURE PAYMENTS
We happily accept a wide range of payment options so you can pay for the things you need in the way that is most convenient for you
* Options may vary by product and licensing agreement. Zoomed Pictures can be adjusted in the Basket.