Chemical Reaction Collection
"Unveiling the Mysteries: The Glowing Dance of Chemical Reactions" Glow
All Professionally Made to Order for Quick Shipping
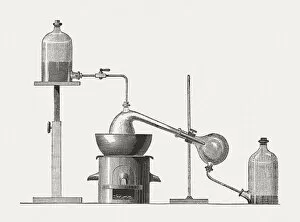
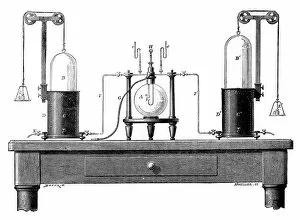
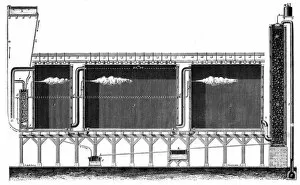
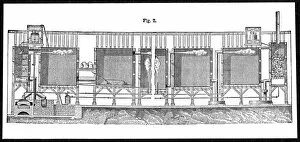
"Unveiling the Mysteries: The Glowing Dance of Chemical Reactions" Glow: Witness the mesmerizing radiance as chemical elements unite in a captivating dance of light and energy. Interaction 1: Explore the intricate web of reactions where atoms collide, bond, and transform, creating new substances with unique properties. Interaction 2: Behold the dynamic interplay between reactants and products, as they engage in a constant exchange of electrons and energy. Preparation of diethyl ether, wood engraving published in 1880: Step back in time to witness the meticulous craftsmanship involved in preparing diethyl ether - an essential compound with diverse applications. Lead chamber for production of sulphuric acid, 1866: Delve into history's industrial revolution as massive lead chambers played their part in producing one of chemistry's most vital compounds - sulphuric acid. Preparation of chlorine water, wood engraving published in 1880: Discover how chlorine water was meticulously prepared through careful chemical processes that harnessed its powerful disinfectant properties. Acid manufacturing, 1832. Artist William Orr: Appreciate the artistic representation capturing early acid manufacturing techniques that paved the way for countless scientific advancements. Antoine Lavoisier's apparatus for synthesizing water from hydrogen (left) and oxygen (right), 1881: Marvel at Lavoisier's groundbreaking apparatus that revealed water's true composition – a milestone moment unveiling nature’s hidden secrets. Sectional view of lead chambers for large-scale production of sulphuric acid, 1870: Peer inside colossal lead chambers which facilitated mass production methods crucial to meet society’s growing demand for sulphuric acid during industrialization. Sectional view of Gay-Lussac's lead chambers and absorption towers, 1870: Witness Gay-Lussac’s innovative design featuring absorption towers, revolutionizing sulphuric acid production and minimizing environmental impact.