Anode Collection
"The Anode: Unveiling the Marvels of Science" Step into the world of scientific wonders as we explore the fascinating concept of anode
All Professionally Made to Order for Quick Shipping
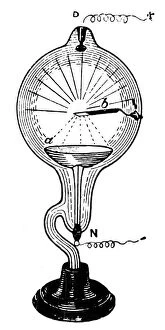
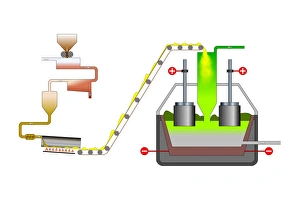
"The Anode: Unveiling the Marvels of Science" Step into the world of scientific wonders as we explore the fascinating concept of anode. From Les Merveilles de la Science, published in 1870, we embark on a journey through various illustrations and discoveries that shed light on this essential component. In one illustration, we witness the Grove cell in action. As electrons from the zinc anode travel through an external circuit to reach the copper electrode acting as a cathode, they engage with hydrogen ions from acid, resulting in the formation of hydrogen gas molecules. This process showcases how anodes play a crucial role in chemical reactions. Delving deeper into its significance, another illustration demonstrates how zinc atoms within an electrode dissolve when exposed to acid. By losing electrons and transforming into cations, these atoms contribute to important reactions facilitated by anodes. Moving forward in time to 1879, we encounter a Crookes tube featuring a strip of platinum serving as an intriguing example of apparatus utilizing anodes for specific purposes within physics experiments. The positive terminal drawing electrons from the copper pipe's anode oxidizes copper atoms into cations further exemplifies their transformative power. The captivating world of technology also embraces this phenomenon with inventions like light-emitting diodes (LEDs) that rely on efficient electron flow between cathodes and anodes to produce mesmerizing illumination. Electroetched Zinc Anode images captured using scanning electron microscopy (SEM) showcase intricate details highlighting its unique properties. Venturing even further back in history to Coolidge X-ray tubes and Crookes cathode ray tubes from the 1880s reveals yet another application for this remarkable component – generating X-rays and exploring mysterious rays emitted by cathodes. But it doesn't stop there. An unexpected connection arises as we learn about electricity production derived from rice plants and sodium hydroxide production processes where specialized electrodes act as critical catalysts for these groundbreaking advancements.